Medicud, a startup focused on wound healing, has raised over €1.3 million ($1.4 million) in seed funding. This includes €766K ($829K) from private investors and €575K ($622K) in grants. In total the company has raised €2.5 million ($2.7 million) since it started.
SUMMARY
- Medicud, a startup focused on wound healing, has raised over €1.3 million ($1.4 million) in seed funding.
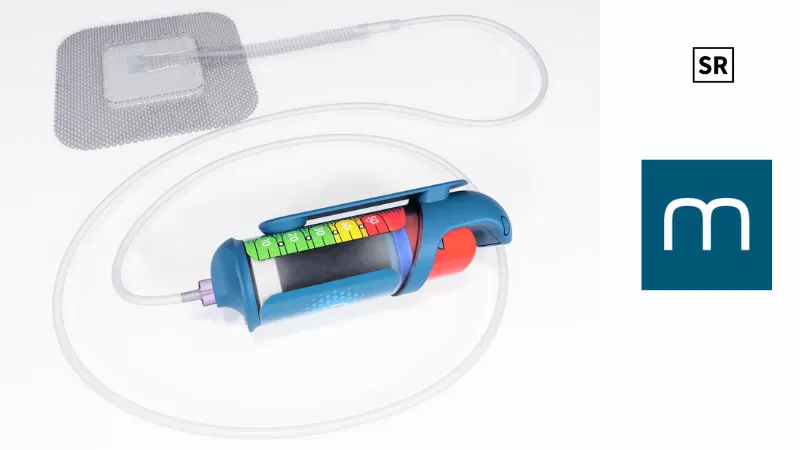
- Medicud is a startup focused on wound healing. Its main product, DRYUM™, is a simple easy-to-use device for surgical wound care.
New investors in this round include US-based CHMBR Partners, 50 Partners Capital Health, BADGE, and several other individual and strategic backers.
The funding will help Medicud begin the approval process for a major clinical trial of its device, DRYUM — the first manually powered negative pressure wound therapy device made for surgical incisions.
Medicud is a startup focused on wound healing. Its main product, DRYUM™, is a simple easy-to-use device for surgical wound care.
RECOMMENDED FOR YOU

Midnite Secures €30M To Expand Its Sportsbook And Casino Platform
Kailee Rainse
Jan 16, 2026
The trial will focus on orthopedic surgeries like hip and knee replacements. It will include 59 patients and take place at Ospedale Pietro e Michele Ferrero in Piedmont, Italy, led by Prof. Marco Schiraldi.
Antonio Nunzio d’Angelo, CEO and co-founder of Medicud said, “I am proud of what we have accomplished at Medicud. The team worked tirelessly to develop the DRYUM device and make it work in the shortest time possible. Clinicians need a new standard of care for wound healing, both cost effective and efficient in reducing surgical complications, and more sustainable, The support and recognition we received from this syndicate of international investors will help accelerate our clinical development and bring us closer to providing clinicians and patients worldwide with a new and innovative solution to post-operative wound healing. Our Series A funding, expected in H1, 2026, will be pivotal for the commercialization of our device.”
DRYUM is a single-use device that uses steady negative pressure to help surgical wounds heal better and reduce complications. Unlike current systems that are bulky, costly, and hard to use, DRYUM is light, easy to handle with one hand, and doesn’t require complex training.
Since it runs manually (not electronically), it’s quieter, more affordable, and easier to access for hospitals and doctors around the world. It also comes with different dressing sizes and shapes to fit various wound types.
Charles Hirschler, Managing Member of CHMBR Partners, LLC said, “We invest in promising companies in sports and health in three areas: biotech, medtech and consumer-facing technologies. Medicud falls squarely within these boundaries. We are delighted to have invested in this company, whose first product demonstrates a high expertise and offers a strong added value with its simplicity, reliability, and modest cost compared to the competition. It opens up a market that was previously not attractive due to excessive pricing and minimal benefits compared to simple bandages,".
Medicud will first focus on the four most common surgeries in the EU and US: C-sections, hip replacements, knee replacements, and breast reconstruction after mastectomy. These make up about 4.66 million procedures each year, with a market worth over €400 million ($433M).
Globally, there are 236 million surgeries every year, creating a market of up to €22 billion ($23.8B) for post-surgery wound care. Over 10% of patients face complications at the surgical site, mainly infections and wound reopening. DRYUM aims to become the go-to solution for preventing these issues.
Read also - EcoDataCenter funding news – EcoDataCenter Secures €450 Million in Funding
Sandrine Egron, Investment Director at 50 Partners Capital Health said, “Medicud is tackling a critical yet often overlooked issue in surgical care: the prevention of post-operative complications through effective wound management. Its innovative approach to portable negative pressure therapy combines medical-grade efficacy with unprecedented usability and affordability, We were impressed by their deep clinical insight, strong execution capabilities and the positive traction they have built with surgeons. At 50 Partners Health, we are proud to support Medicud’s journey toward transforming surgical recovery across Europe and beyond.”
The funding will also help Medicud get regulatory approvals for DRYUM, including the CE mark in Europe and FDA approval in the US, which they aim to complete by mid-2026. These steps support the company's plan to expand globally.
DRYUM is classified as a class IIa device in Europe and a class II device in the US eligible for a simpler 510(k) approval process. Medicud also plans to grow its team by hiring a commercial director.
About Medicud
Medicud is a startup focused on wound healing. Its main product, DRYUM™, is a simple easy-to-use device for surgical wound care. It’s the only ultra-portable, mechanical system that gives steady negative pressure throughout treatment. DRYUM is designed to reduce common post-surgery issues like infections and wound reopening, aiming to set a new standard in healing.


 Follow us
Follow us Follow us
Follow us