[Funding alert] Manchester-based Phagenesis Secures $42 Million in Series D Funding
Mar 5, 2024 | By Startup Rise EU
Manchester-based Phagenesis secures $42 million in series D funding. When brain injury (including stroke) or extended mechanical ventilation cause disruptions to the neurological components of swallowing coordination and control, the Phagenyx® neurostimulation system targets and restores those functions.
Dysphagia, or trouble swallowing, prevents patients from eating, drinking, or controlling their own saliva in a safe or efficient manner. Dysphagia is linked to significantly greater healthcare expenses and can frequently result in potentially fatal complications like pneumonia. The investment is mainly meant to assist commercialization in the US and increase penetration in Europe, given that the FDA just approved Phagenyx® in the US.
Read also - Cayman Islands-based Ether.fi Secures $23Million in Series A Round Funding
Additionally, it will help with regulatory actions, clinical trials, and pipeline product development. Sectoral and EQT Life Sciences led the joint investment syndicate from the United States and Europe. Aphelion, Northern Gritstone, and British Patient Capital were among the new investors who joined the round.
RECOMMENDED FOR YOU
According to Reinhard Krickl, CEO of Phagenesis: “This investment from a highly experienced international investor syndicate will accelerate access to and adoption of our therapy. We will invest in exceptional talent to scale up our customer outreach and to support passionate clinicians who want to bring our therapy to those who need it. Our novel and proven therapy can help millions of patients who suffer from swallowing disorders.”
Drew Burdon, Partner at EQT Life Sciences, said: “Dysphagia is a severe medical condition that affects countless patients in hospital. It can increase the hospital length of stay, the risk of complications, and lengthen recovery time. The Phagenyx System demonstrates significant reductions in hospital length of stay and corresponding and substantial reduction in healthcare costs, as evidenced by the Company’s strong portfolio of high-quality clinical studies. This strongly aligns with EQT’s Health Economics strategy of transforming cutting-edge scientific innovation into impactful and cost-effective healthcare solutions. We‘re excited to support the next phase of Phagenesis’ journey.”
Michael Sjöström, Co-Founder and Partner at Sectoral Asset Management, added: “Dysphagia-associated complications are known to increase mortality substantially, but there is still a lack of adequate treatment modalities. That’s why Phagenyx is an amazing innovation with the potential to change care paradigms. It allows treatment of dysphagia very early in the care pathway, which prevents later complications. Our investment will support the Phagenesis team in making Phagenyx more broadly available, specifically in the United States.”
About Phagenesis
They at Phagenesis are committed to revolutionizing the treatment of dysphagia. Millions of individuals worldwide suffer from the crippling condition known as dysphagia, or trouble swallowing. They created the Phagenyx, a ground-breaking device that treats dysphagia using pharyngeal electrical stimulation (PES), because they think everyone should be able to enjoy their food and live life to the fullest.
Read also - Utrecht-based CryoCloud Secures €500k in Pre-Seed Funding
Recommended Stories for You

SERDA Therapeutics funding news – Amsterdam-based SERDA Therapeutics Secures €2Million in Funding
Startup Rise EU Jun 4, 2024

AccountsIQ funding news – Dublin-based AccountsIQ Secures €60Million in Series C Round Funding
Startup Rise EU Jun 13, 2024

[Funding alert] Belgium-based Tree Energy Solutions Secures €140Million in Funding
Startup Rise EU Apr 16, 2024

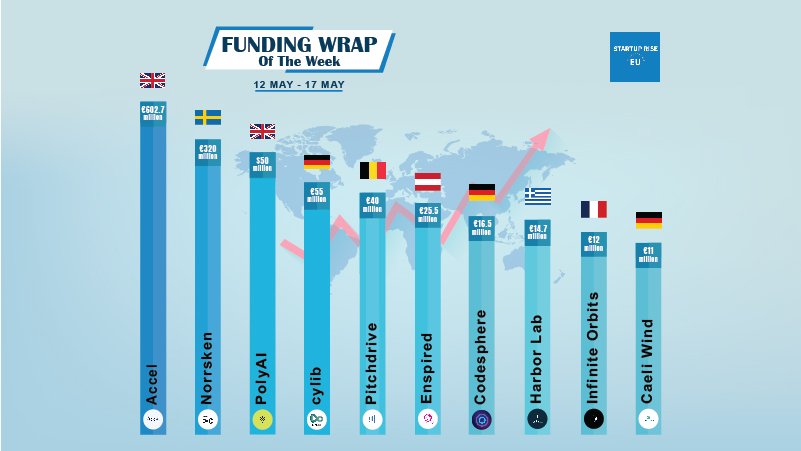
Codesphere funding news – Karlsruhe-based Codesphere Secures €16.5 million in Funding
Startup Rise EU May 17, 2024

SurrealDB Funding News – Uk-based SurrealDB Raises $20Mn to Disrupt Database Tech
Team SR Jun 19, 2024

[Funding alert] Paris-based Rayon Secures €4 million in seed funding
Startup Rise EU Sep 27, 2023


 Follow us
Follow us Follow us
Follow us